Search Results
Results for: 'Chemistry tutorial-Ch63-3-Precipitation titration: using indicator to determine the end point (Mohr's method)'
Chemistry Experiment - 32.3 - Investigating Effect of Electrodes on Products of Electrolysis
By: t1709, Views: 1523
Chemistry Experiment - 32.3 - Investigating Effect of Electrodes on Products of Electrolysis
Chemistry Experiment - Ch01 - 02 - Reaction of Copper with Nitric Acid
By: t1709, Views: 1531
Chemistry Experiment - Ch01 - 02 - Reaction of Copper with Nitric Acid
By: t1709, Views: 1603
Chemistry Experiment - 32.2 - Investigating Effect of Concentration on Preferential Discharge of Ions
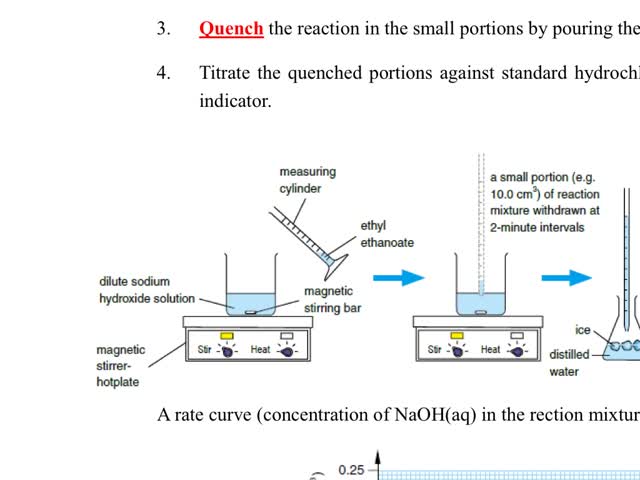
Chemistry tutotial-Ch36-7-Follow the progress of reaction by titmetric analysis
By: t0605, Views: 772
Chemistry tutotial-Ch36-7-Follow the progress of reaction by titmetric analysis
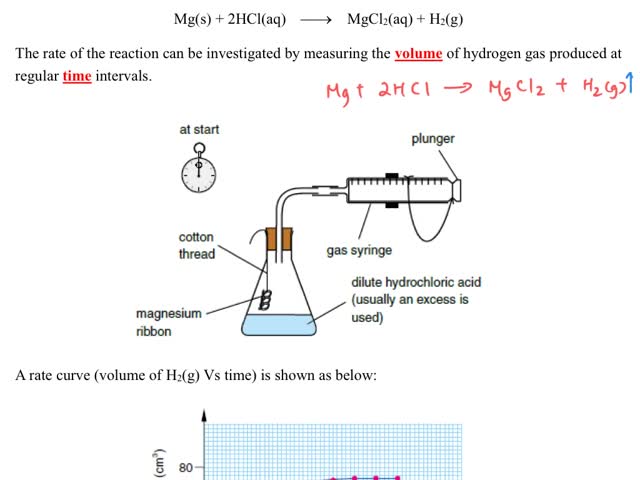
Chemistry tutotial-Ch36-3-Follow the progress of reaction by measuing the change in volume of gases
By: t0605, Views: 1064
Chemistry tutotial-Ch36-3-Follow the progress of reaction by measuing the change in volume of gases
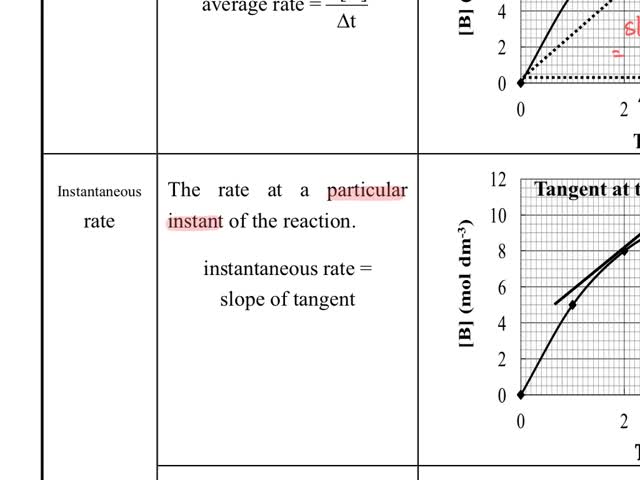
Chemistry tutotial-Ch36-2-Average rate and instantaneous rate
By: t0605, Views: 773
Chemistry tutotial-Ch36-2-Average rate and instantaneous rate
By: t0605, Views: 1145
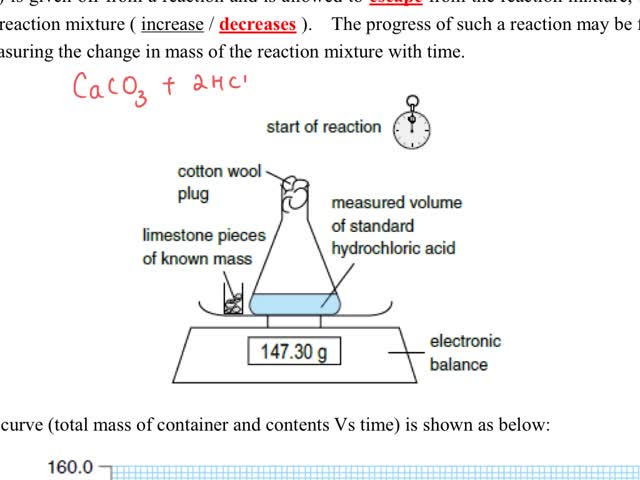
Chemistry tutotial-Ch36-5-Follow the progress of reaction by measuing the change in mass of reaction mixture
By: t0605, Views: 1006
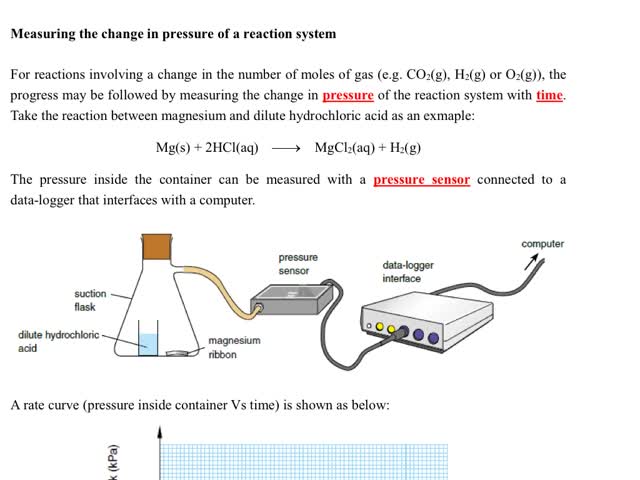
Chemistry tutotial-Ch36-4-Follow the progress of reaction by measuing the change in pressure of reaction mixture
By: t0605, Views: 811
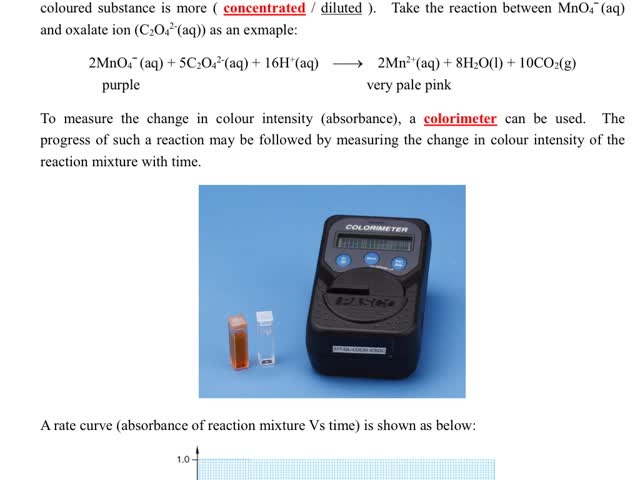
Chemistry tutotial-Ch36-6-Follow the progress of reaction by measuing the change in colour intensity of reaction mixture
YU CHUN KEUNG MEMORIAL COLLEGE