Search Results
Results for: 'Chemistry tutorial-Ch30-12-Sulphite ion as a reducing agent'
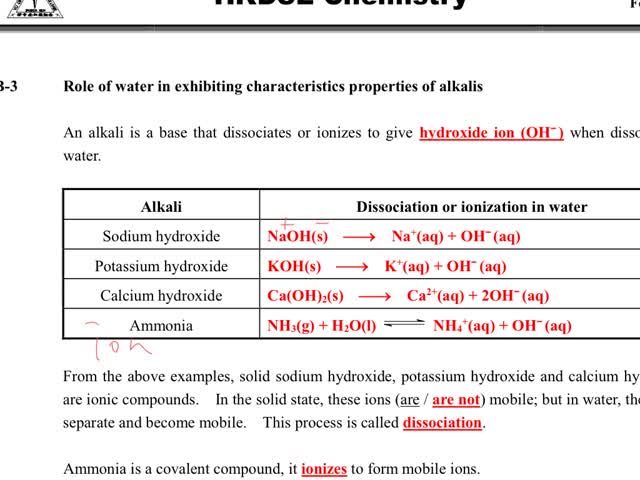
Chemistry tutorial-Ch14-7-Role of water in exhibiting characteristics properties of alkalis
By: t0605, Views: 1317
Chemistry tutorial-Ch14-7-Role of water in exhibiting characteristics properties of alkalis
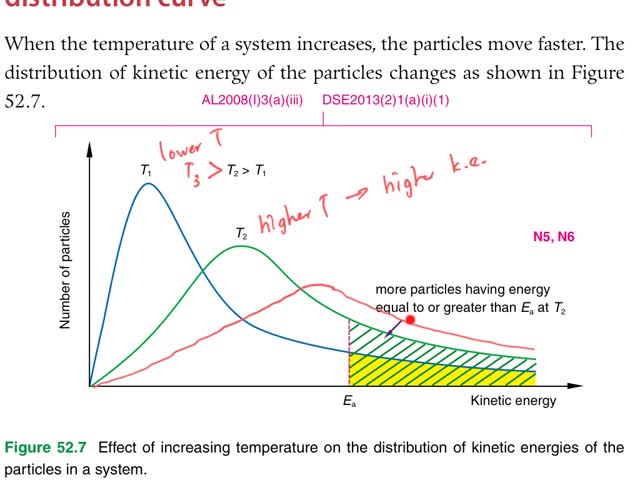
Chemistry tutorial-Ch52-4-Explaining effect of temperature change on reaction rate
By: t0605, Views: 1331
Chemistry tutorial-Ch52-4-Explaining effect of temperature change on reaction rate
By: t0605, Views: 1274
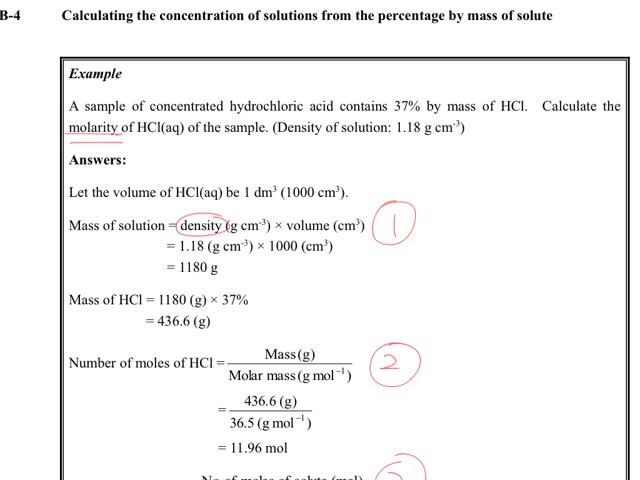
Chemistry tutorial-Ch15-6-Calculating the concentration of solutions from the percentage by mass of solute
By: t0605, Views: 1239
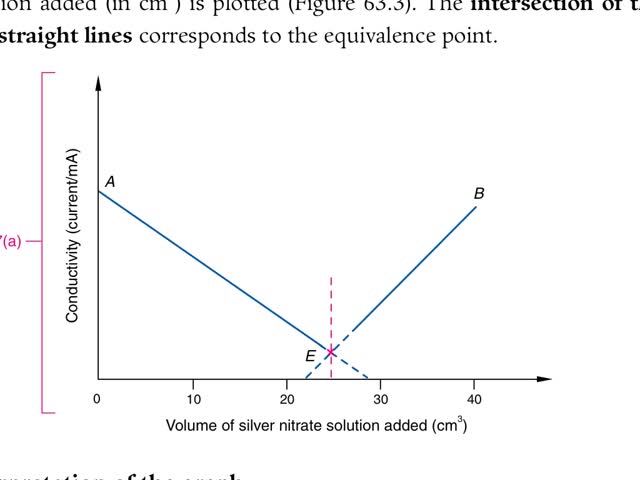
Chemistry tutorial-Ch63-2-Precipitation titration: using conductivity method to determine the end point
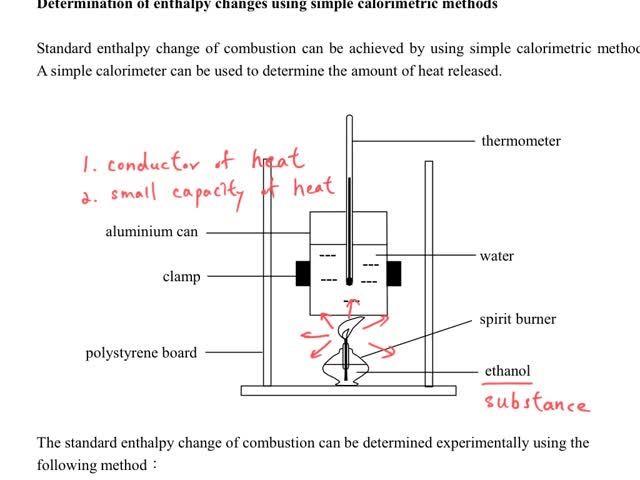
Chemistry tutorial-Ch34-6-Calorimetric method for measuring standard enthalpy change of combustion
By: t0605, Views: 1736
Chemistry tutorial-Ch34-6-Calorimetric method for measuring standard enthalpy change of combustion
By: t0605, Views: 1608
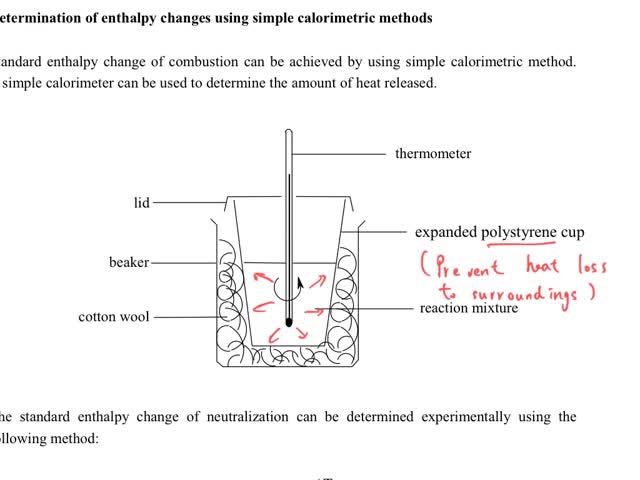
Chemistry tutorial-Ch34-8-Calorimetric method for measuring standard enthalpy change of neutralization
By: t0605, Views: 1765
Chemistry tutorial-Ch63-3-Precipitation titration: using indicator to determine the end point (Mohr's method)
Biological concepts Ch.9 (10) - Adaptation of leave for reducing water loss
By: t0906, Views: 1189
Biological concepts Ch.9 (10) - Adaptation of leave for reducing water loss
By: t0605, Views: 1559
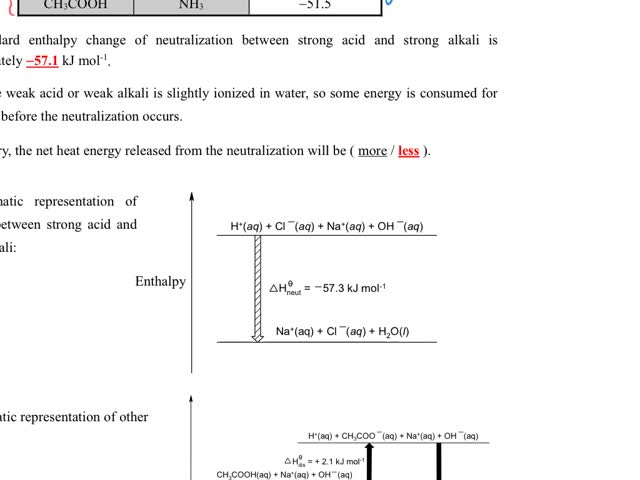
Chemistry tutorial-Ch34-9-Affect of the strength of acids or bases on the standard enthalpy change of neutralization
YU CHUN KEUNG MEMORIAL COLLEGE